Density of the gas phase. Variable fixed Shape.
Solved Describe The Relative Densities Of The Phases For Chegg Com
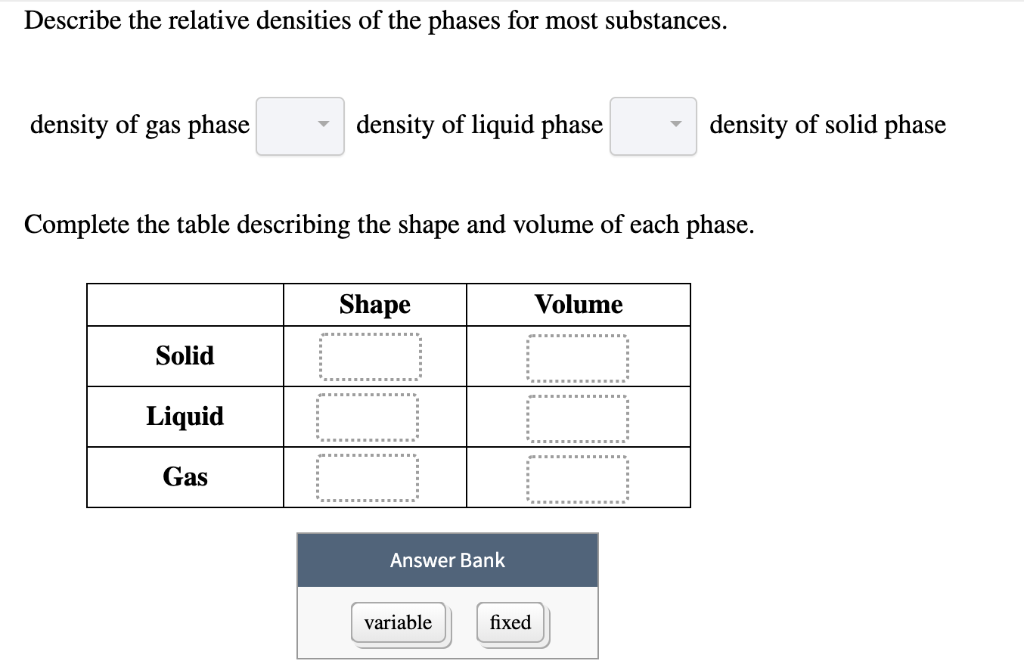
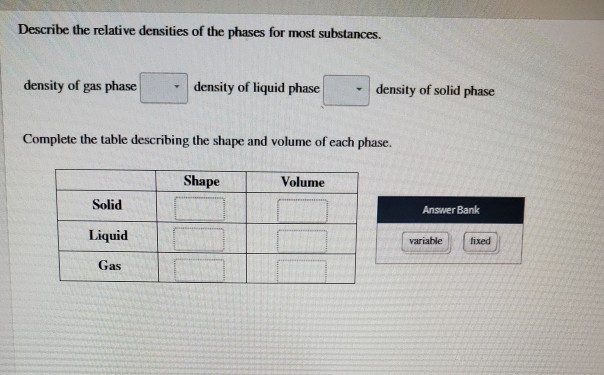
Describe the relative densities of the phases for most substances.

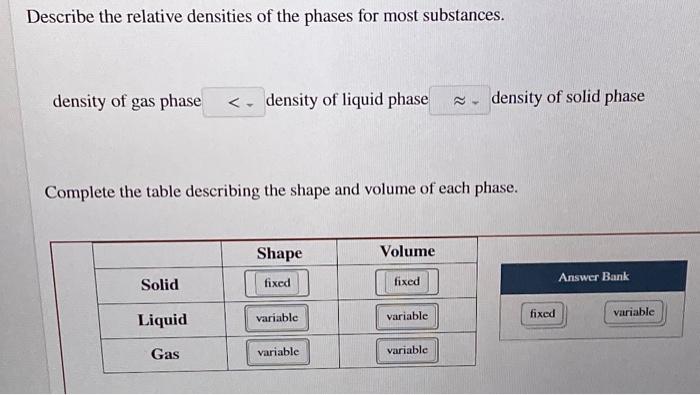
. When water freezes the molecules do not stack into a close-packed structure. Complete this table describing the shape and volume of each phase. -In a phase diagram the solid-liquid coexistence line has a negative slope-The solid is less dense than the liquid-The molecules are closest in the liquid phase Most other substances-High pressure will cause the liquid to become solid-The solid is more dense than the liquid-The molecules are closest in the solid phase.
Or density of solid phase 2 Complete this table describing the shape and volume of each phase. Describe the relative densities of the phases for most substances. Most substances are densest as a solid because when they are cooled their particles contract.
8 density of the gas phase density of the o density of the O solid phase uid phase 2 Complete this table describing the shape and volume of each phase. For most substances the solid phase is most dense and the gas phase is least dense. Shape Volume Answer Bank Solid Liquid ied variable Gas.
Describe the relative densities of the phases for most substances. Density of the gas phase. This is the best answer based on feedback and ratings.
Show transcribed image text. The atoms are closely spaced and hence the density is high. Density of gas phase __density of liquid phase ____ density of solid phase.
Density of liquid phase density of solid phase Complete the table describing the shape and volume of each phase. Or to density of liquid phase is. Under normal ambient conditions water is less dense as a solid than as a liquid so ice floats on water.
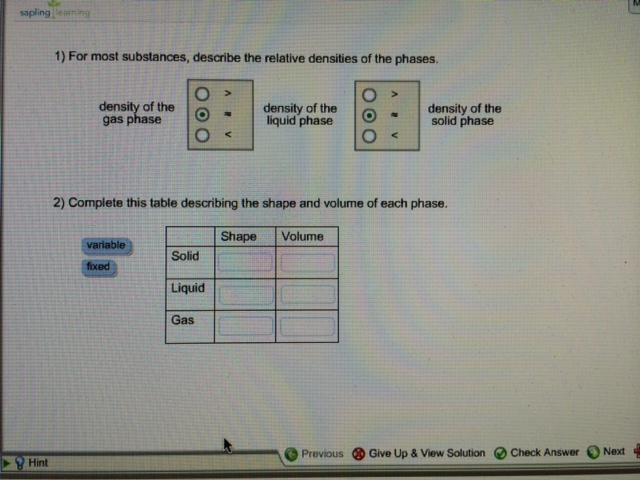
For most substances describe the relative densities of the phases. Describe the relative densities of the phases for most. Describe the relative densities of the phases for most substances.
Because it is a ratio relative density or specific gravity is a unitless value. Classify these images as representing a solid liquid or gas at the molecular level. This is why ICE F L O A T S even though its a solid.
Density of gas phase. Density of gas phase density of liquid phase density of solid phase Complete the table describing the shape and volume of each phase. However WATER is the exception to this rule.
Density is a measure of the amount of mass contained in a unit of volume. The general trend is that most gases are less dense than liquids which are in turn less dense than solids but there are numerous exceptions. There are three main phases of matter.
It is also known as specific gravity SG. 1 For most substances describe the relative densities of the phases. Moving along a constant temperature line reveals relative densities of the phases.
Relative density RD is the ratio of the density of a substance to the density of water. 1 For most substances describe the relative densities of the phases. Moving along a constant pressure line reveals relative energies of the phases.
Now up your study game with Learn mode. Density of the gas phase density of the liquid phase density of the solid phase 2 Complete this table describing the shape and volume of each phase Shape Volume fixed Solid. Answer to Describe the relative densities of the phases for most substances.
1 For most substances describe the relative densities of the phases. You just studied 8 terms. Heres a table of densities of common substances including several gases liquids and solids.
The relative densities of water ice and alcohol are 10 09 and 08 respectively. Density of gas phase - density of liquid phase density of solid phase Complete the table describing the shape and volume of each phase. 1 For most substances describe the relative densities of the phases.
Describe the shape vol of each phase. When moving from the bottom of the diagram to the top the relative density increases. Students also viewed these chemistry questions.
For most substances describe the relative densities of the phases. Describe what will happen to the substance when it begins in a vaccum at -15C and is slowly. Uestion 3 of 12 Describe the relative densities of the phases for most substances.
Solids consist of atoms that are arranged in a regular crystal lattice. The relative densities of water ice and alcohol are 10 09 and. Water reaches its greatest density at 398 degrees Celcius - in the LIQUID PHASE.
Solid - Liquid - Gas - 1 For most substances describe the relative densities of the phases. Solid - Liquid - Gas -Volume. Density of gas phase is.
Density of the gas phase. Density of gas phase - density of. For most substances describe the relative densities of the phases.
If its value is less than 1 then the substance is less dense than water and would float. Updated on March 18 2019. Most materials are more dense as solids.
Heat would cause an increase in volume which would cause a decrease in density. Read also relative and learn more manual guide in describe the relative densities of the phases for most substances 8 density of. Shape Volume Solid fixed fixed Answer Bank.
Solved For Most Substances Describe The Relative Densities Chegg Com
Solved Describe The Relative Densities Of The Phases For Chegg Com
Solved Describe The Relative Densities Of The Phases For Chegg Com
0 Comments